Pentamethylcyclopentadienyl rhodium dichloride dimer
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Di-μ-chloro-bis[chloro(pentamethylcyclopentadienyl)rhodium(III)] | |
| Other names Dichloro(pentamethylcyclopentadienyl)rhodium(III) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
InChI
| |
| |
| Properties | |
Chemical formula | C20H30Cl4Rh2 |
| Molar mass | 618.07 g·mol−1 |
| Appearance | red solid |
Solubility in water | dichloromethane, chloroform |
| Hazards | |
| GHS labelling: | |
Pictograms |   |
| Danger | |
Hazard statements | H302, H312, H315, H319, H332, H334, H335 |
Precautionary statements | P261, P264, P270, P271, P280, P285, P301+P312, P302+P352, P304+P312, P304+P340, P304+P341, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P342+P311, P362, P363, P403+P233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Pentamethylcyclopentadienyl rhodium dichloride dimer is an organometallic compound with the formula [(C5(CH3)5RhCl2)]2, commonly abbreviated [Cp*RhCl2]2 This dark red air-stable diamagnetic solid is a reagent in organometallic chemistry.[1]
Structure and preparation
The compound has idealized C2h symmetry. Each metal centre is pseudo-octahedral.
The compound is prepared by the reaction of rhodium trichloride trihydrate and pentamethylcyclopentadiene in hot methanol, from which the product precipitates:[1]
- 2 C5(CH3)5H + 2 RhCl3(H2O)3 → [(C5(CH3)5)RhCl2]2 + 2 HCl + 6 H2O
It was first prepared by the reaction of hydrated rhodium trichloride with hexamethyl Dewar benzene[2]
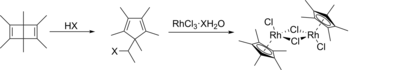
This complex was first prepared from hexamethyl Dewar benzene and RhCl3(H2O)3.[3][4][5] The hydrohalic acid necessary for the ring-contraction rearrangement is generated in situ in methanolic solutions of the rhodium salt, and the second step has been carried out separately, confirming this mechanistic description.[6] The reaction occurs with the formation of 1,1-dimethoxyethane, CH3CH(OCH3)2, and hexamethylbenzene is produced by a side reaction.[5][6]
This rhodium(III) dimer can be reduced with zinc in the presence of CO to produce the rhodium(I) complex [Cp*Rh(CO)2].[7]
Reactions
Reductive carbonylation gives [Cp*Rh(CO)2].[8]
The Rh-μ-Cl bonds are labile and cleave en route to a variety of adducts of the general formula Cp*RhCl2L. Treatment with silver ions in polar coordinating solvents causes precipitation of silver(I) chloride, leaving a solution containing dications of the form [Cp*RhL3]2+ (L = H2O, MeCN).
The chemistry is similar to that of the analog pentamethylcyclopentadienyl iridium dichloride dimer.
Further reading (early literature)
- Kang, Jung W.; Mosley, K.; Maitlis, Peter M. (1968). "Mechanisms of Reactions of Dewar Hexamethylbenzene with Rhodium and Iridium Chlorides". Chem. Commun. (21): 1304–1305. doi:10.1039/C19680001304.
- Kang, Jung W.; Maitlis, Peter M. (1968). "Conversion of Dewar Hexamethylbenzene to Pentamethylcyclopentadienylrhodium(III) Chloride". J. Am. Chem. Soc. 90 (12): 3259–3261. doi:10.1021/ja01014a063.
- Criegee, Rudolf; Grüner, H. (1968). "Acid-catalyzed Rearrangements of Hexamethyl-prismane and Hexamethyl-Dewar-benzene". Angew. Chem. Int. Ed. 7 (6): 467–468. doi:10.1002/anie.196804672.
- Kang, Jung W.; Moseley, K.; Maitlis, Peter M. (1969). "Pentamethylcyclopentadienylrhodium and -iridium halides. I. Synthesis and properties". J. Am. Chem. Soc. 91 (22): 5970–5977. doi:10.1021/ja01050a008.
- Herrmann, Wolfgang A.; Zybill, Christian (1996). "Bis{(μ-chloro)[chloro(η-pentamethylcyclopentadienyl)rhodium]} — {Rh(μ-Cl)Cl[η-C5(CH3)5]}2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 148–149. ISBN 9783131791610.
- Heck, Richard F. (1974). "Reactions of Dienes Trienes and Tetraenes with Transition Metal Compounds". Organotransition Metal Chemistry: A Mechanistic Approach. Academic Press. pp. 116–117. ISBN 9780323154703.
References
- ^ a b White, C.; Yates, A.; Maitlis, Peter M. (2007). "(η 5 -Pentamethylcyclopentadienyl)Rhodium and -Iridium Compounds". Inorganic Syntheses. Vol. 29. pp. 228–234. doi:10.1002/9780470132609.ch53. ISBN 9780470132609.
{{cite book}}:|journal=ignored (help) - ^ Paquette, Leo A.; Krow, Grant R. (1968). "Electrophilic Additions to Hexamethyldewarbenzene". Tetrahedron Lett. 9 (17): 2139–2142. doi:10.1016/S0040-4039(00)89761-0.
- ^ Paquette, Leo A.; Krow, Grant R. (1968). "Electrophilic Additions to Hexamethyldewarbenzene". Tetrahedron Lett. 9 (17): 2139–2142. doi:10.1016/S0040-4039(00)89761-0.
- ^ Criegee, Rudolf; Grüner, H. (1968). "Acid-catalyzed Rearrangements of Hexamethyl-prismane and Hexamethyl-Dewar-benzene". Angew. Chem. Int. Ed. 7 (6): 467–468. doi:10.1002/anie.196804672.
- ^ a b Herrmann, Wolfgang A.; Zybill, Christian (1996). "Bis{(μ-chloro)[chloro(η-pentamethylcyclopentadienyl)rhodium]} — {Rh(μ-Cl)Cl[η-C5(CH3)5]}2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 148–149. ISBN 9783131791610.
- ^ a b Heck, Richard F. (1974). "Reactions of Dienes Trienes and Tetraenes with Transition Metal Compounds". Organotransition Metal Chemistry: A Mechanistic Approach. Academic Press. pp. 116–117. ISBN 9780323154703.
- ^ Herrmann, Wolfgang A.; Zybill, Christian (1996). "Dicarbonyl(η-pentamethylcyclopentadienyl)rhodium — Rh[η-C5(CH3)5](CO)2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 147–148. ISBN 9783131791610.
- ^ Herrmann, Wolfgang A.; Zybill, Christian (1996). "Dicarbonyl(η-pentamethylcyclopentadienyl)rhodium — Rh[η-C5(CH3)5](CO)2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 147–148. ISBN 9783131791610.
- v
- t
- e
| Organorhodium(0) compounds |
|
|---|
- RhCl(P(C6H5)3)3
| Organorhodium(I) compounds |
|
|---|
- Rh2(CH3CO2)4
| Organorhodium(II) compunds |
|
|---|
- RhBr3
- Rh(C5H5N)4Cl3
- RhCl3
- Rh(ClO4)3
- RhF3
- Rh(OH)3
- RhI3
- Rh(NH3)5ClCl2
- Rh(NO3)3
- Rh2O3
- Rh2S3
- Rh2(SO4)3
- YbRh2Si2
| Organorhodium(III) compunds |
|---|
- RhO2
- RhF4
- RhF5
- XeRhF6
- RhF6











