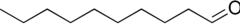
Decanal
 | |
| Names | |
|---|---|
| Preferred IUPAC name Decanal | |
| Other names Decyl aldehyde, caprinaldehyde | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.003.598 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C10H20O |
| Molar mass | 156.269 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.83 g/mL |
| Melting point | 7 °C (45 °F; 280 K) |
| Boiling point | 207 to 209 °C (405 to 408 °F; 480 to 482 K) |
| Hazards | |
| GHS labelling: | |
Pictograms |  |
| Warning | |
Hazard statements | H315, H319, H412 |
| P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P501 | |
| NFPA 704 (fire diamond) |  2 2 0 |
| Flash point | 85 °C (185 °F; 358 K) |
Autoignition temperature | 200 °C (392 °F; 473 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 3730 mg/kg (rat, oral) 5040 mg/kg (rabbit, dermal) |
| Safety data sheet (SDS) | Fisher Scientific |
| Related compounds | |
Related compounds | 2-Decanone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Decanal is an organic compound classified as an aldehyde with the chemical formula C10H20O.
Occurrence
Decanal occurs naturally in citrus, along with octanal, citral, and sinensal, in buckwheat,[1] and in coriander essential oil.[2] It is used in fragrances and flavoring.[3]
Preparation
Decanal can be prepared by oxidation of the related alcohol decanol.[4]
Safety
For safety information see the MSDS.[5]
References
- ^ Janes D, Kantar D, Kreft S, Prosen H (2008). "Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC-MS". Food Chemistry. 112: 120–124. doi:10.1016/j.foodchem.2008.05.048.
- ^ Nurzyńska-Wierdak, Renata (2013). "Essential oil composition of the coriander (Coriandrum sativum L.) herb depending on the development stage". Acta Agrobotanica. 66: 53–60. doi:10.5586/aa.2013.006.
- ^ Rychlik, Schieberle & Grosch (1998). Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants. Lichtenbergstraße, Germany.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ R. W. Ratcliffe (1988). "Oxidation with the Chromium Trioxide-Pridine Complex Prepared in situ: 1- Decanal". Organic Syntheses; Collected Volumes, vol. 6, p. 373.
- ^ "Safety (MSDS) data for n-decanal". Archived from the original on 2004-05-20. Retrieved 2007-12-01.









